Your Trusted Partner in Medical Moulding Excellence
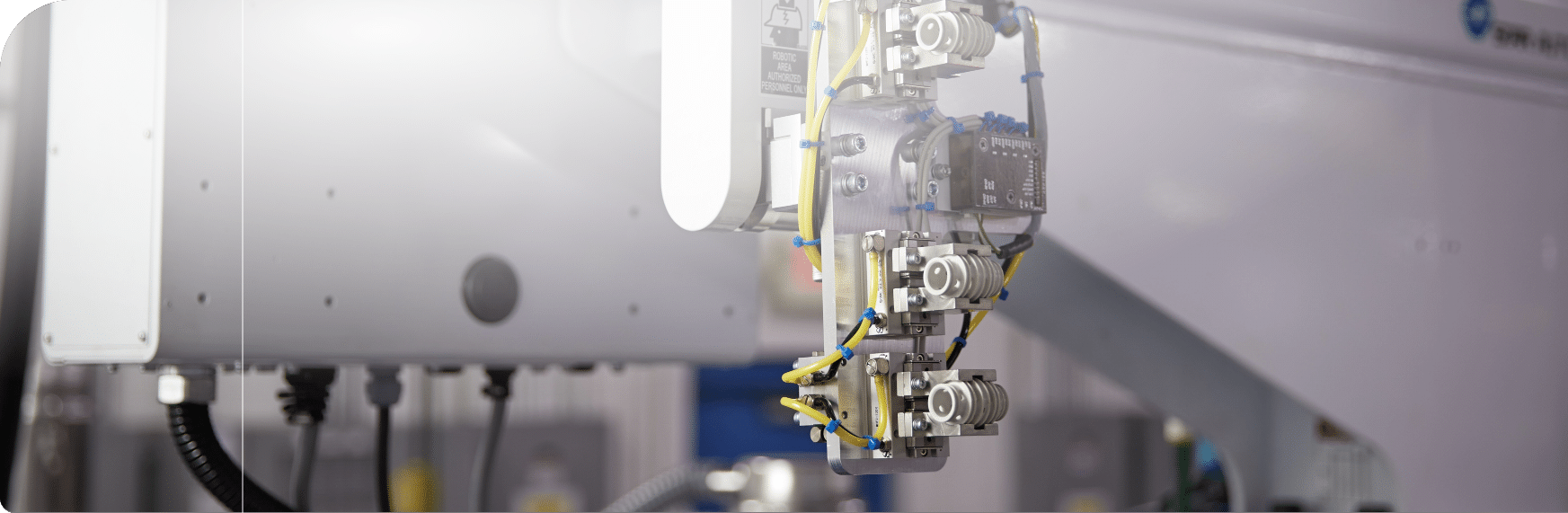
At Rosti, we specialise in delivering high-quality, precision-moulded components for the medical and pharmaceutical industries. With a strong focus on compliance, sustainability, and innovation, we support customers from concept to reality. Our industry‑leading clean‑room certified injection moulding capabilities are specifically designed for highly regulated medical and diagnostic applications. With Class 7 & 8 cleanrooms located globally and full ISO 13485 certification, our facilities provide the precision, consistency, and quality you can trust in every component we produce.
Our unique value lies in:
- Customer-centric approach: Partnerships with leading medical brands to create long-term value
- End-to-end support: From design and prototyping to full-scale production
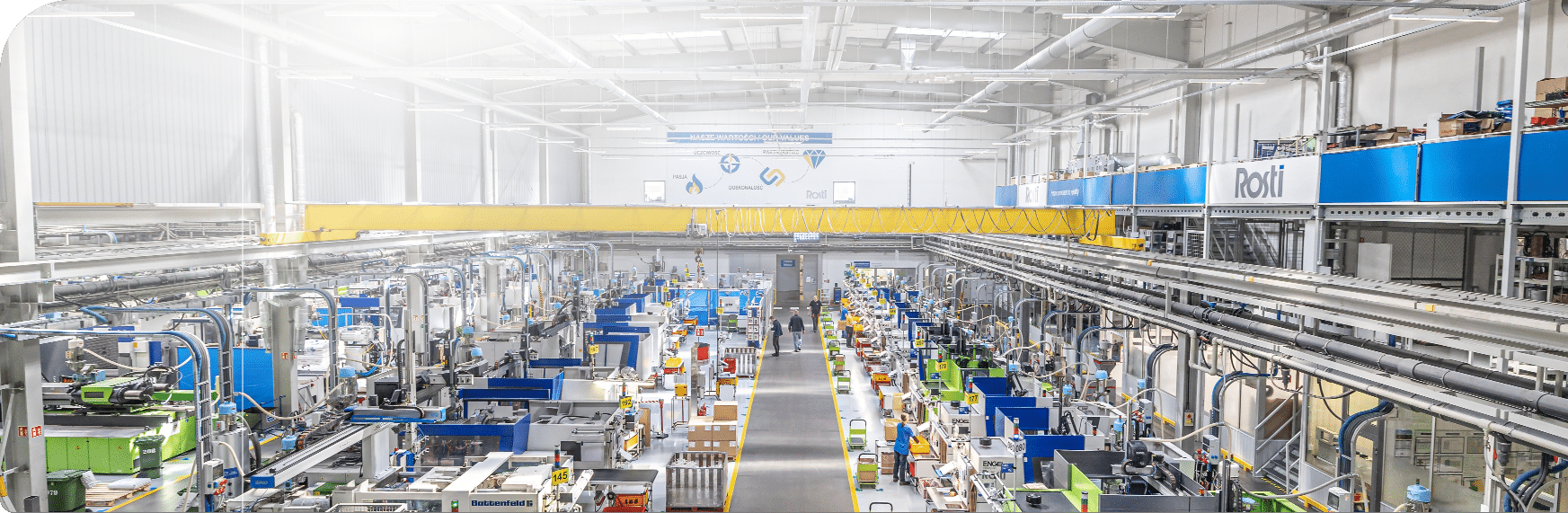
- Global footprint: Production worldwide in North America, Europe and Asia, makes it possible for us to support partners in multiple locations
- Cleanroom Production: Cleanroom class 7 & 8 production areas spread out globally
- Relentless focus on quality: Quality awareness built in our DNA over decades with a unified global QMS
- Strong track record for Innovation and Engineering: Strong innovation and engineering power via our 3 innovation centres provides technical solution offering for specific customer challenges
- Sustainability mindset: Compliant with CSRD and ISO standards
We understand the critical nature of medical applications and deliver solutions that meet the highest standards of safety, reliability, and traceability.
Serving the World’s Most Trusted Medical Brands
Rosti supports OEMs and Tier 1 suppliers across a wide spectrum of medical and pharmaceutical applications, including:
- Medical devices
- Diagnostics and lab consumables
- Pharmaceutical packaging
- Consumer health products
Our customers include global leaders who rely on Rosti for quality, reliability, and innovation. These partnerships reflect our commitment to excellence and our ability to scale with our customers’ needs.
Advanced Capabilities for Critical Applications
Rosti’s medical manufacturing capabilities are designed to meet the stringent demands of the healthcare industry. We offer:
- Class 7 & 8 Cleanrooms globally for sterile and particle-controlled environments
- ISO 13485 certified production for medical device quality assurance
- Precision injection moulding using advanced tooling and automation
- Automated assembly and testing for consistent quality and efficiency
- Tooling and prototyping to accelerate product development
- Traceability and validation systems to ensure compliance and transparency
Our facilities are equipped to handle complex projects, tight tolerances, and high-volume production with full regulatory compliance.
Certified for Confidence
Rosti’s commitment to quality and compliance is reflected in our certifications:
These certifications ensure that our processes meet international standards and that our customers can trust the integrity of every component we produce.
Our medical manufacturing facilities meet the rigorous requirements of ISO 13485, an international standard for quality management systems in the medical device industry.
We maintain certified cleanroom facilities in accordance with ISO 14644-1, which establishes standards for air cleanliness in cleanrooms and controlled environments.
Our production environment ranges from 'white room' to certified cleanroom class7 & 8, ensuring that we can meet the specific requirements of your medical device
Our NPI procedure covers both new product development and existing product transfers, with defined gates, start conditions, scope, and goals overseen by a steering group.
All of our tools, equipment, and production processes are validated through Rosti's standard validation routine with IQ, OQ, PQ, or according to specific customer requirements.

Thought Leadership in Medical Moulding
We regularly share insights and updates to help our customers stay informed and inspired. Explore our latest content:
- Global Insight: Medical Moulding by Gustav Reingsdahl – A deep dive into trends and technologies shaping the future of medical plastics
- Cleanroom Investment in Rosti Poland – Expanding our capabilities to meet growing demand
- Blog posts on sustainability, compliance, and innovation in medical manufacturing
This section will be continuously updated to reflect new developments, investments, and thought leadership.
Frequently Asked Questions
What truly distinguishes our Medical division is its commitment to surpassing regulatory standards. With a keen focus on stringent requirements such as the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) in Europe, our team of dedicated professionals ensures that our processes and products consistently meet and exceed industry expectations.
As well, when dealing with our Medical Solutions Team, you’ll have access to a team entirely dedicated to bring your medical device to live from concept to reality. From R&D to project management and production, our Medical Solutions Team can support you at any step of the process.
The Medical Solutions division ensures a Customer Focused and tailored approach to every single project to achieve the highest quality standards as well as meeting Customer’s deadlines.
As a leading CMO, our Medical division delivers a comprehensive suite of services underpinned by regulatory expertise, innovation, and sustainability focus.
At the core of our value proposition lies our deep understanding of regulatory frameworks, ensuring compliance with the highest standards of safety and efficacy. Through strategic investments in R&D, we continuously push the boundaries of innovation, collaborating closely with our customers to translate ideas into tangible solutions. Whether it’s developing technologies or optimizing existing designs for manufacturability, our team stands ready to deliver innovation at every stage of the product lifecycle.
Our commitment to sustainability extends beyond mere compliance, encompassing a proactive approach to environmental stewardship, and embracing a networked approach to tackle emissions. From light weighting initiatives to sustainable materials and designs for recyclability, we strive to offer sustainable solutions to our customers to minimize our environmental footprint while maximizing value for our customers.
Through collaborative problem-solving and a focus on manufacturability, we empower our customers to realize their vision and drive progress in the healthcare industry. With a global footprint and scalable capabilities, we stand ready to support their expansion into new markets and ensure continuity of supply on long-life cycle programs. By functioning as a critical supply chain partner, we enable our customers to focus on what they do best – driving innovation and improving patients’ lives.
In the fast-paced world of the medical industry, our innovation centres play a pivotal role in supporting our customers to stay ahead of the curve.
Key for Rosti is to remain agile in today’s fast-paced, ever-evolving development cycles. That’s why Rosti is one of the few companies equipped to harness the latest advances in 3D printing. By utilising Carbon® M3 printers and Digital Light Synthesis printing technology, we reduce lead times for prototype part testing, empowering our customers to accelerate their time to market. Integration of 3D printing with traditional injection moulding allows for rapid prototyping and the development of more complex designs and geometries.
Our dedicated innovation team collaborates closely with customers, offering solutions early in the development process to increase manufacturability and create additional value.
We stay ahead of industry trends by investing in R&D and adhering to technical roadmaps inspired by NASA technology readiness levels (TRLs). This ensures our readiness to address evolving customer needs while maintaining close relationships with suppliers.
In conclusion, our innovation centres serve as the engine driving our commitment to excellence and continuous improvement. By embracing cutting-edge technologies, fostering collaboration, and staying attuned to market dynamics, we support our customers in navigating the complexities of the medical industry with confidence and agility. As we look to the future, we remain steadfast in our dedication to pushing boundaries, delivering innovation, and driving value for our customers and partners alike.
We operate Class 7 & 8 cleanrooms globally (currently in China, Poland, Turkey, and USA), suitable for a wide range of medical and diagnostic applications.
Yes, our medical production facilities are ISO 13485 certified, ensuring compliance with global medical device standards.
Absolutely. We offer design, tooling, and prototyping services to help customers accelerate development and reduce time-to-market.
We work with a wide range of medical-grade thermoplastics, including bio-based and recyclable options to support sustainability goals.
Our production systems include full traceability and validation protocols, ensuring every part meets regulatory and customer requirements.
Introducing
Inside Rosti Medical Solutions

Get in touch
We are only a message away.
Our expertise and global footprint, over sites strategically placed in the Europe, Asia and USA, make Rosti your local plastic injection moulding partner.
Contact us today and one of our team will respond to your enquiry.