Nutritional Feeding sets are critical in modern healthcare, enabling the safe and effective delivery of fluids, medications, and nutrients to patients. These devices comprise multiple precision-engineered components, each of which must work together seamlessly to guarantee safety and performance.
For more than 15 years, Rosti has partnered with a leading medical customer to manufacture high-volume IV sets, ensuring consistent quality, compliance with strict medical regulations, and reliable supply to global markets. This long-term collaboration showcases our ability to combine engineering expertise, contract manufacturing, and logistics to support customer growth on a global scale.
The Customer Challenge
When the customer first approached Rosti, they were facing a number of challenges:
- Supplier quality concerns: Their existing European supplier had quality issues that posted concerns related reliability.
- No technical data – Drawings and component specifications were not available, leaving the customer vulnerable to inconsistencies.
- Ageing tooling: Old tooling limited scalability and risked compromising product consistency.
Our customer needed a partner who could reverse engineer the entire Nutritional Feeding set, recreate missing technical data, and provide a long-term manufacturing solution capable of scaling up to tens of millions of units annually.
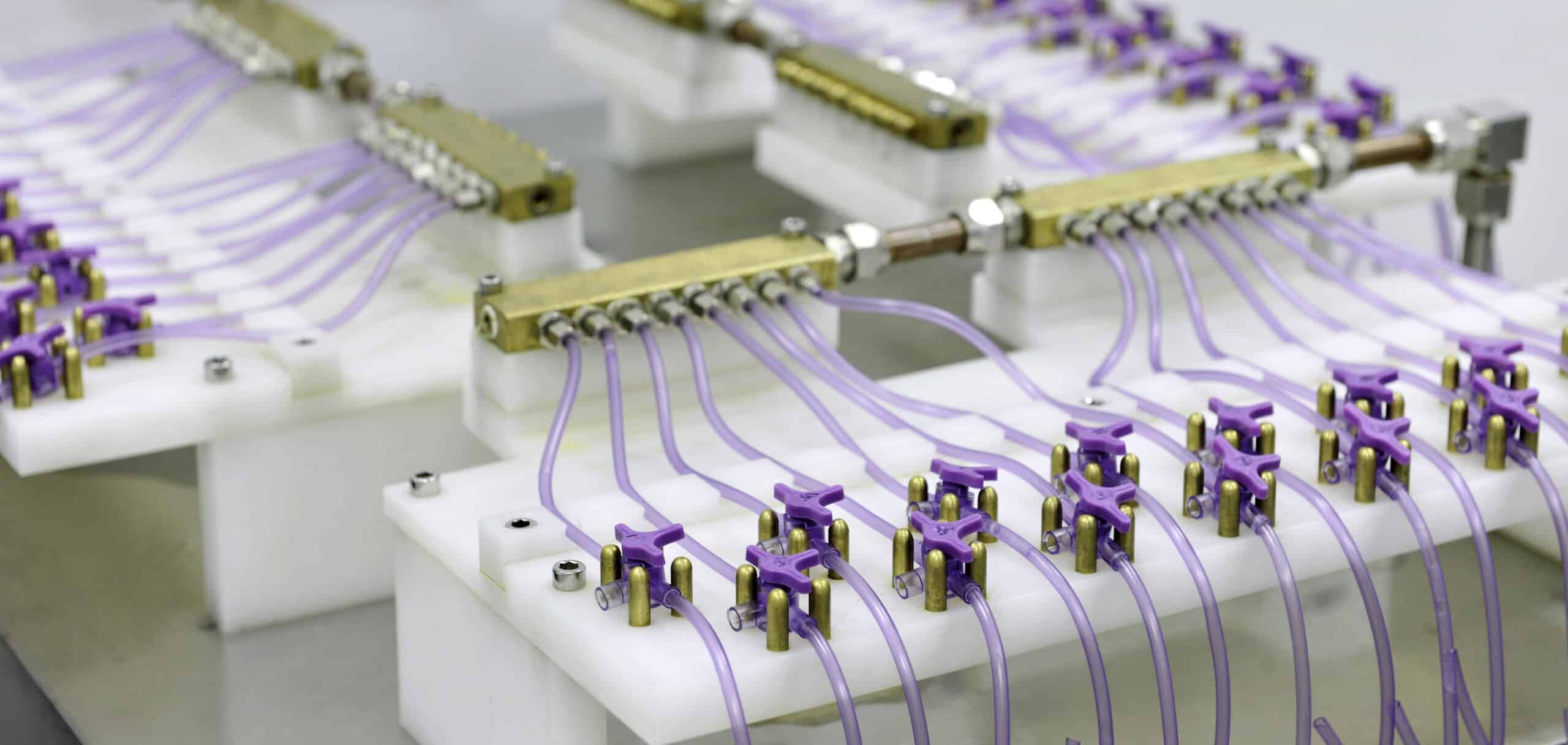
Feeding Set Components
The IV set comprises multiple components, all of which must integrate precisely to maintain safety and function –
- Stopcocks – control fluid flow
- Tubing connectors – ensuring secure connections
Rosti’s role was to ensure that each part was replicated with absolute accuracy and manufactured to the highest medical standards, while also allowing for future material and design improvements.
Scaling Production to Meet Demand
From the outset of the partnership, the customer’s objective was to secure a stable, scalable supply chain to meet rising global demand.
Today, Rosti manufactures between 50 and 60 million feeding sets per year, supplying both European and Asian markets. Over the years, production has steadily increased in line with the customer’s growth, supported by Rosti’s ability to scale facilities, implement lean manufacturing principles, and maintain strict quality management systems.
This scale-up demonstrates not only the resilience of our operations but also its commitment to supporting customers’ long-term success.

The Reverse Engineering Process
With no technical drawings or CAD models available, our task was to rebuild the nutritional feeding set from the ground up. The new components had to be identical to the originals to avoid disrupting functionality in clinical use.
Key Milestones
- CT scanning of original parts to generate a high-resolution point cloud model.
- CMM analysis to capture critical dimensions and tolerances.
- CAD model creation based on the scan data and measurements.
- Design review using digital simulation tools, such as Moldflow, to optimise manufacturability and identify any improvements.
- Customer collaboration to review and approve the CAD models.
- Tooling design and manufacture to enable high-volume production.
- Medical validation and regulatory approval prior to mass production.
Timeline
- Reverse engineering and digital modelling – completed within weeks.
- Full implementation, tooling, and validation – approximately 1.5 years.
This process not only recreated the original components but also provided the customer with a complete set of technical drawings and digital models – an asset they had never previously owned.
Material Development and Transitions
Over the course of the partnership, materials have evolved in response to both regulatory changes and cost pressures:
- Initial phase: materials matched the original supplier’s specification to ensure continuity.
- First transition: a change was made to comply with updated medical regulations.
- Second transition: optimisation for cost reduction while maintaining compliance and performance.
Rosti’s ability to manage these transitions smoothly reflects both its materials expertise and its commitment to balancing compliance, performance, and cost-effectiveness.
Contract Manufacturing & Logistics
This project highlights the value of Rosti’s full contract manufacturing offering:
- Design & engineering – from reverse engineering to CAD modelling and simulation.
- Tooling & validation – partnered with tool maker to construct tools, followed by rigorous medical validation.
- High-volume production – producing tens of millions of sets annually under ISO-certified conditions.
- Logistics & distribution – shipping to both European and Asian markets, ensuring a reliable global supply chain.
By handling the full process from design through to delivery, Rosti provided a seamless and fully integrated solution.
Results & Impact
- Improved quality: consistent, validated manufacturing processes solved previous supplier issues.
- Full ownership: customer gained digital drawings and specifications for the first time.
- Scalable supply chain: production scaled up to 50–60 million sets annually.
- Long-term partnership: Rosti has supported the customer for over 15 years and continues to evolve the product and materials.
This has positioned Rosti as a strategic partner driving growth, reliability, and innovation.
This case study highlights how Rosti’s technical expertise, medical validation capabilities, and global footprint enabled a customer to overcome supplier challenges, secure a robust supply chain, and scale production to meet growing demand worldwide.
By combining reverse engineering, material development, and high-volume manufacturing, Rosti has become a trusted partner in the delivery of safe, high-quality nutritional feeding sets to healthcare providers across Europe and Asia.